Super-resolution Fluorescence Microscopy
Selected projects performed at ScopeM using super-resolution fluorescence microscopy techniques are listed below.
The thrust for deciphering molecular or biological processes at the nanoscale has led to the development of a variety of microscopy techniques. On one hand, optical and fluorescence microscopy provides specificity, minimal invasiveness and the possibility to monitor processes in living specimens and in real time. However, fluorescence microscopy was originally hampered by the diffraction of light that limits the resolution to approx. half of the wavelength of light (typically 250 nm in the visible spectrum).
This “traditional” limit has been overcome in the last years by the development of optical super-resolution microscopy techniques, such as STORM (Rust, Bates, & Zhuang, 2006), PALM (Betzig, et al., 2006), SIM (Gustafsson, 2000) and STED (Hein, Willig, & and Hell, 2008). Some of these techniques can reach a resolution limit down to tenths of nanometers. The development of such, as they are called, nanoscopy techniques has pioneered research in biology, but also in chemistry, materials science and recently in physics and their inventors have been awarded the Nobel prize in Chemistry in 2014.
The term SMLM includes basically three techniques, which were independently developed in 2006. They presented an elegant way to sequentially excite, stochastically read out and precisely localize multiple single fluorescent molecules in labeled specimens. The molecules used for labeling are photoswitchable dyes and fluorescent proteins. These imaging techniques are:
- stochastic optical reconstruction microscopy (STORM)
- photoactivated localization microscopy (PALM)
- fluorescence photoactivation localization microscopy (FPALM)
The common fundamental principle behind all three techniques is that the majority of the fluorescent molecules are in a long-lasting and stable "dark" or "OFF" state. Then either an UV laser is used for activation of a small number of these fluorescent probes which are then excited by a second laser, or this excitation is done purely stochastically. Once a fluorescent probe is excited, it emits photons during a so-called "ON" state. The total number of photons emitted is crucial as we will see below.
The second essential condition is that the molecules which are in their "ON" state should be separated with each other by a distance that exceeds the Abbe diffraction limit (greater than approx. 250 nm). After photon emission the molecule will either enter again into the "OFF" state or it will photobleach. These cycles of "ON" and "OFF" can eventually be repeated for thousands of times while a single-photon sensitive camera is recording images. Therefore, in each camera frame there will be only a sparse subset of fluorophores active ("ON").
Finally, these thousands of camera images are processed by a dedicated software to find the exact spatial coordinates (positions) of each individual fluorescent emitter. The precision of these localizations (known as localization precision) is the most crucial aspect in SMLM and depends strongly on the number of photons emitted by each molecule, as given by the formula of Thompson (Thompson, Larson, & Webb, 2002). In other words, the localization precision is limited only by the number of photons detected for each individual fluorescent emitter.
Once all the molecule coordinates are determined, the final step is their visualization, where all the positions are converted into an actual specimen image. With this technique and our N-STORM microscope we can routinely achieve spatial resolution down to 20-30 nm.
Examples
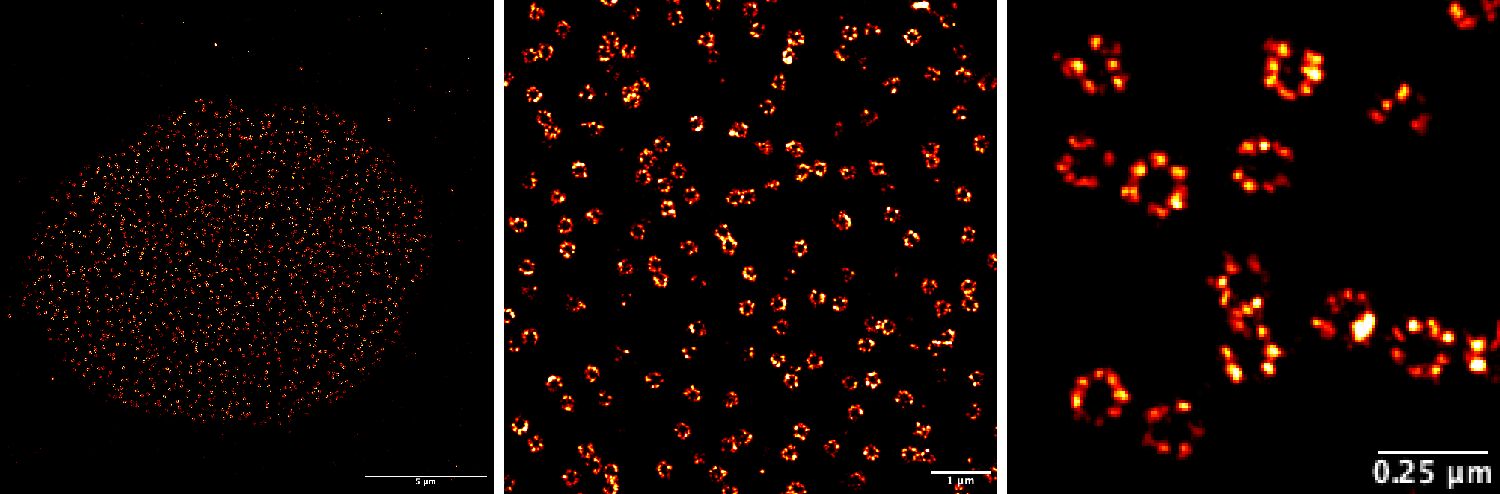
The figure below shows an example of our recent super-resolution by Single Molecule Localization Microscopy images of Nuclear Pore Complexes (NPCs) in mammalian cells obtained with our N-STORM microscope, by Roja Gandhimathi () and Dorothea Pinotsi () depicting the resolution power of the technique.
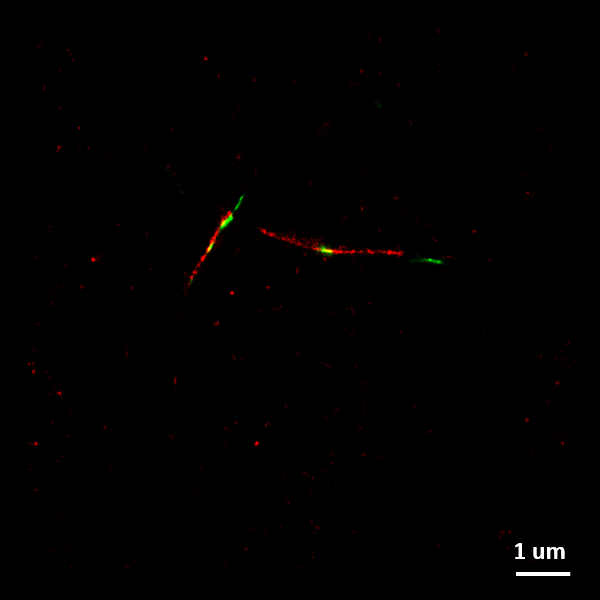
The figure to the right shows a SMLM image of two-color alpha-synuclein amyloid fibrils. Fibrillar seeds (labelled in green) elongated by monomeric protein (labelled red) to form these two-color fibrils.
Image credit: Dorothea Pinotsi
All images were obtained with the Nikon N-STORM system at ScopeM.